|
| | | | | | | |
|
Immunosuppressants and Antibiotics Bayesian dose adjustment
Pharmacokinetics Newsletter
LIMOGES, Thursday 20th
December 2018
|
|

Web Portal
|

Seasons Greetings
The TDM team
|
¤ In 2019, the ISBA website will be
replaced by a new solution called Optim'IS, developed by the Company
Optim'Care (an English version will of course be available).
|
|
 New
characteristics and fonctions:
New
characteristics and fonctions:
-
Medical device with the CE marking
-
Certified «healthcare datacentre»
-
Possibility of secured online drug
prescription
-
New «draft» request status in order
to facilitate data sharing by the health professionals (e.g., transplant
physician and local clinical pharmacologist).
-
Simplified request form
-
Possibility of secured online
exchanges between requesters and pharmacologists at Limoges to make it
possible to share additional information about requests and results.
The platform will also provide other
solutions (telemonitoring, e-learning for patients …) to help transplant
physicians improve patient care
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
This message is not a spam
but has been sent to you because you have requested information from the
Pharmacology and Toxicology Laboratory at the Limoges University Hospital.
If
you wish to be withdrawn from this mailing list, please send a message to the
following email address: stp@chu-limoges.fr
|
|
Ligne vide invisible pour laisser de la place |
|
|
Adaptation Bayésienne des ImmunoSuppresseurs,
Antibiotiques et Anti-cancéreux
La NEWSLETTER Pharmacocinétique
LIMOGES, le jeudi 5 Octobre 2017
|
|

Portail WEB
|
Le site ABIS fête sa
100.000ème demande !
Chers
utilisateurs du site ABIS, nous venons de valider la 100.000ème
demande d’adaptation d’immunosuppresseurs, depuis avril 2005. Prochain
objectif la 200.000ème !
Merci
beaucoup pour votre confiance!
L’équipe STP
|
|
Ce
mail n'est pas un Spam, vous recevez cette lettre car vous avez demandé à
recevoir des informations du laboratoire de Pharmaco-Toxicologie
du CHU de Limoges.
Si vous voulez être retiré de notre liste de diffusion : stp@chu-limoges.fr
|
|
Immunosuppressants and Antibiotics Bayesian dose adjustment
The Pharmacokinetics Newsletter
LIMOGES, Thursday 5th October
2017
|
|

WEB Portal
|
The ISBA website celebrates its
100,000th request!
Dear ISBA users, we just validated the 100,000th
request for immunosuppressant dose individualization, since April 2005. On
the road to the 200,000th!
Thank you
so much for your trust!
The TDM Team
|
|
This message is not a spam but has been
sent to you because you have requested information from the Pharmacology and Toxicology Laboratory at the
Limoges University Hospital.
If you wish
to be withdrawn from this mailing list, please send a message to the following
email address: stp@chu-limoges.fr
|
|
Ligne vide invisible pour laisser de la place |
|
|
Immunosuppressants and Antibiotics Bayesian dose adjustment
The Pharmacokinetics Newsletter
LIMOGES, Friday 12th May 2017
|
|

WEB Portal
|
Hello !
We are pleased
to announce the publication of a new article about population pharmacokinetic
modeling and Bayesian estimation, which this
time is about ENVARSUS® (Once-daily Tacrolimus) in renal and
hepatic transplantation:
The
ISBA platform is thriving and the number of registered transplantation
centers is steadily increasing, while the platform’s activity has remained
constant during the past year.
We
are taking this opportunity to provide you with a quick overview of the
website activity:
- 9836
immunosuppressant kinetics have been modeled so far this year, coming from 50
different hospital centers!
- Among
them, the three most active are:

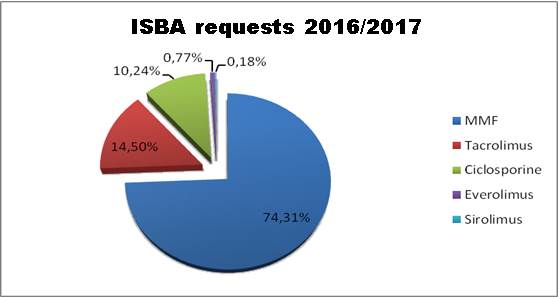
Mycophenolic acid remains the most
modeled immunosuppressant with about three quarters of the total number of
requests:
The
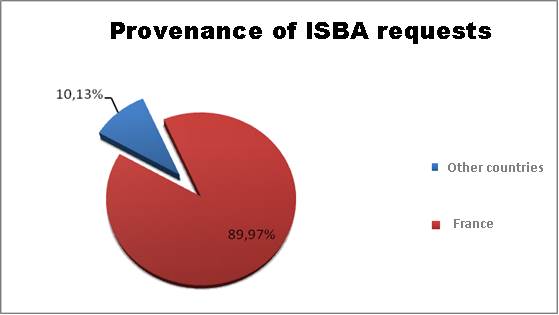
requests mainly come from metropolitan France and its overseas territories. About
10% of the requests are made by other countries, notably North Africa and the
United States of America, or neighbouring countries such as Belgium and
Switzerland!
The ergonomics
of the web portal has been improved, and an ISBA mobile application will be
launched in the near future. The interface will also be improved and a
question-and-answer forum will be set up.
Thank you for your trust !
The TDM Team
|
|
This message is not a spam but has been sent to you because you
have requested information from the Pharmacology and Toxicology Laboratory at
the Limoges University Hospital.
If you wish to be withdrawn from this mailing list, please
send a message to the following email address: stp@chu-limoges.fr
|
|
Ligne vide invisible pour laisser de la place |
|
|
Immunosuppressants and Antibiotics Bayesian dose adjustment
The Pharmacokinetics Newsletter
LIMOGES, Tuesday 27th December
2016
|
|

WEB Portal
|
¤
The ISBA website has expanded this year :
-
Population pharmacokinetic models and Bayesian
estimators have been developed for tacrolimus (Envarsus®
formulation) in kidney and liver transplantation (optimal sampling times 0, 8
and 12h).
-
Population pharmacokinetic models and Bayesian
estimators have been developed for tacrolimus (Advagraf®
formulation) in liver transplantation (optimal sampling times 0, 2 and 6h).
¤
Among our New Year resolutions, we are planning to offer you :
-
A new and more ergonomic homepage, adapted for a
variety of screens (tablet, fixed
navigator, …)
-
A question and answer forum for problems when
entering data on ISBA ( coming summer 2017)
-
The development of PKJust
(dose adjustment of antibiotics), taking into account biological and
bacteriological information so as to personalize the therapeutic target.
Thank you for your trust !
The TDM Team
|
|
Seasons Greetings to you all
|
|
This message is not a spam but has
been sent to you because you have requested information from the Pharmacology and Toxicology Laboratory at the
Limoges University Hospital.
If you wish
to be withdrawn from this mailing list, please send a message to the following
email address: stp@chu-limoges.fr
|
|
Ligne vide invisible pour laisser de la place |
|
|
Immunosuppressants Bayesian Dose Adjustment
The I.S.B.A NEWSLETTER
Tuesday 28th June 2016
|
|

ISBA
|
New Bayesian estimators
We
are pleased to inform you that new Bayesian estimators dedicated to the
tacrolimus formulation Envarsus® in adult kidney and liver
transplantation are now available on our ISBA website.
Envarsus®
is a once-daily prolonged release formulation of tacrolimus. It is
characterized by increased intestinal bioavailability owing to the
Meltdose® technology. This leads to the administration of smaller doses
(about 30% less) in comparison to other tacrolimus formulations. Since
October 2014, Envarsus® has been approved in Europe for the prevention of
rejection in kidney or liver transplantation or the treatment of rejection
resistant to other immunosuppressants in adult transplant patients.
Veloxis
and Chiesi provided us with the pharmacokinetic data of phase 2 clinical
trials in renal and hepatic transplantation to allow us to develop population
pharmacokinetic models and Bayesian estimators and we express our gratitude
to them.
This
tacrolimus formulation is characterized by a different pharmacokinetic
profile as compared to Advagraf® with, in particular, lower maximal
concentration (the figure below gives examples of modeled PK profiles in
renal transplantation).
The
slow and prolonged release of Envarsus® leads to a different limited
sampling strategy for accurate Bayesian estimation of the AUC0-24h: 0, 8, 12h
after dose intake for both types of transplantation.
Of
note, it is the best limited sampling strategy leading to the lowest bias
between predicted and observed AUC0-24h. However, AUC0-24h
can be estimated from samples taken at (slightly) different times on
condition that the exact times are known.
In conclusion, these new tools are
available for adult renal and hepatic transplantation whatever the period
post transplantation and the associated immunosupressants.
¤ Examples of PK
profiles observed and modeled
Figure
1-Examples of PK profiles modeled using 3 samples (0, 8, 12h) (in black) and
observed (in blue) in adult renal transplant patients in a validation dataset
(data splitting).
      
Thank you for your trust!
The TDM Team
|
|
This message is not a spam
but has been sent to you because you have requested information from the
Pharmacology and Toxicology Laboratory at the Limoges University Hospital.
If you wish to be withdrawn from this mailing list, please send a message to
the following email address: stp@chu-limoges.fr
|
|
Ligne vide invisible pour laisser de la place |
|
| |
| | | | |
|
| |
| |
|
|
| |